California NORML has provided a great summary of MMRSA (Medical Marijuana Regulation Safety Act). For the first time in 20 years the State of California is going to begin fully regulating medical cannabis. Read the summary below:
The new Medical Marijuana Regulation and Safety Act consists of three separate bills which were enacted together on Sept 11, 2015 (despite its title, the term “medical cannabis” is used throughout the act). The bill creates a comprehensive state licensing system for the commercial cultivation, manufacture, retail sale, transport, distribution, delivery, and testing of medical cannabis. All licenses must also be approved by local governments.
The law will take effect on Jan 1, 2016. After that, the state will need several months (probably at least a year) to set up the necessary agencies, information systems, and regulations to actually begin issuing licenses. In the interim, local governments may choose to adopt new ordinances to permit or license local businesses in preparation for state licensing. Facilities currently operating in accordance with state and local laws may continue to do so until such time as their license applications are approved or denied. In the meantime, prospective applicants are strongly advised to apply to the state Board of Equalization for a Resale Permit, and to prepare for seeking approval from their local governments.
Text of Medical Marijuana Regulation Safety Act (three parts):
AB 266 (Bonta/Cooley/Jones-Sawyer/Lackey)
AB 243 (Wood)
SB 643 (McGuire)
SUMMARY:
AGENCIES AB 266 establishes a new Bureau of Medical Marijuana Regulation under the Department of Consumer Affairs. The Bureau is to establish a comprehensive internet system for keeping track of licensees and reporting the movement of commercial cannabis and cannabis products.
SB 643 & AB 243 give the Dept. of Food and Agriculture responsibility for regulating cultivation; the Dept. of Public Health for developing standards for manufacture, testing, and production and labeling of edibles; the Dept of Pesticide Regulation for developing pesticide standards; and the Depts. of Fish and Wildlife and State Water Board for protecting water quality. (Sec. 19332)
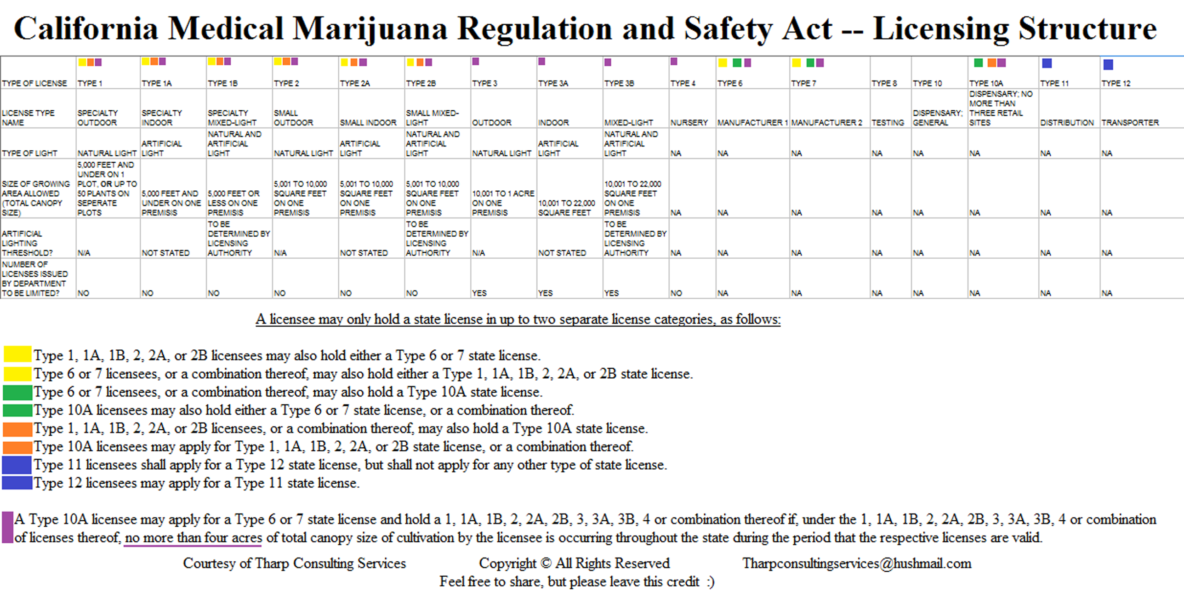
LICENSE TYPES The following license types are established under AB 266 (19300.7)) and SB 643 (19331(g)):
(a) Type 1 = Cultivation; Specialty outdoor. Up to 5,000 square ft of canopy, or up to 50 noncontiguous plants
(b) Type 1A = Cultivation; Specialty indoor. Up to 5000 sq ft
(c) Type 1B = Cultivation; Specialty mixed-light. Using exclusively artificial lighting.
(d) Type 2 = Cultivation; Outdoor. Up to 5000 sq ft, using a combination of artificial and natural lighting
(e) Type 2A = Cultivation; Indoor. 5001 -10,000 sq ft.
(f) Type 2B = Cultivation; Mixed-light. 5001 -10,000 sq ft
(g) Type 3 = Cultivation; Outdoor. 10,001 sq ft – 1 Acre
(h) Type 3A = Cultivation; Indoor.. 10,001 – 22,000 sq ft
(i) Type 3B = Cultivation; Mixed-light. 10,001 – 22,000 sq ft
(j) Type 4 = Cultivation; Nursery.
(k) Type 6 = Manufacturer 1 for products not using volatile solvents.
(l) Type 7 = Manufacturer 2 for products using volatile solvents.
(m) Type 8 = Testing
(n) Type 10 = Dispensary; General
(o) Type 10A = Dispensary; No more than three retail sites
(p) Type 11 = Distribution
(q) Type 12 = Transporter
CULTIVATION SIZE LIMITATIONS The maximum allowable size is 1 acre (43,560 sq ft) outdoors (Type 3) or 22,000 sq ft indoors (Type 3A and 3B licenses). The DFA is directed to limit the number of Type 3, 3A and 3B licenses. (AB 243, 19332(g)).
VERTICAL INTEGRATION There are complicated restrictions to prevent vertical integration (AB 266, 19328). In general, licensees can only hold licenses in up to two separate categories. Small cultivation licensee Types 1 -2 may hold manufacturing or Type 10A retail licenses (limited to three dispensaries). It appears that Types 3-4 licensees can’t apply for manufacturing licenses at all. However, Type 10A licensees can apply for both manufacturing and cultivation licenses, provided their total cultivation area doesn’t exceed 4 acres. Also, facilities in jurisdictions that require or permit cultivation, manufacture and distribution to be integrated as of July 1, 2015 may continue to operate that way until Jan 1, 2026.
DISTRIBUTORS REQUIRED Type 11 distributors are a new kind of entity that has been created to regulate the flow of products. ALL cultivation and manufacturing licensees are required to send their products to a Type 11 licensee for quality insurance and inspection before passing them to the next stage of manufacturing or retailing. The Type 11 licensee in turn submits the product to a Type 8 laboratory for batch testing and certification. Afterwards, the sample returns to the Type 11 distributor for final inspection and execution of the contract between the cultivator and manufacturer or manufacturer and retailer. The Type 11 distributor charges a fee that covers the testing plus any applicable taxes (the act doesn’t impose any new taxes, but anticipates that could happen in the near future) (AB 266, 19326)
Type 11 distributors and Type 8 testing facilities can’t hold any other kind of licenses (however, licensees may have their own labs for in-house testing).
LOCAL PERMITS REQUIRED No person shall engage in commercial activity without BOTH a state license and a license, permit, or other authorization from their local government. (AB 266, 19320(a); AB 243, 11362.777 (b)).
LAWFUL ACTS Actions by licensees that are permitted by both a state license and local government are lawful and protected from arrest, prosecution, or other legal sanctions (AB 266, 19317).
GRANDFATHERING Facilities already operating in compliance with local ordinances and other laws on or before Jan 1, 2018 may continue to operate until such time as their license is approved or denied. (AB 266, 19321(c)). Facilities in operation before Jan 1, 2016 shall receive priority. Los Angeles may in any case continue to prosecute violations of Measure D.
APPLICANT QUALIFICATIONS (SB 643, 19322): Applicants must provide proof of local approval and evidence of legal right to occupy proposed location. Applicants shall submit fingerprints for DOJ background check. Cultivation licensees must declare selves “agricultural employers” as defined by Alatore-Zenovich-Dunlap-Berman Agricultural Labor Relations Act.
Licensing authority MAY deny application if applicant has been convicted of an offense substantially related to qualifications, including ANY felony controlled substance offense, violent or serious felonies, or felonies involving fraud, deceit or embezzlement, or any sanctions by a local licensing authority in the past 3 years (SB 643, 19323(a)5).
FOR-PROFIT ENTITIES are implicitly allowed under the qualifications established above. These were previously “not authorized” under SB 420, but the new licensing provisions extend to individuals, partnerships, corporations, business trusts, etc. (under the definition of “person” in AB266, 19300.5 (aj)). Likewise, applicants no longer need be patients.
CULTIVATION LICENSING The DFA shall establish a medical cannabis cultivation program. All cultivation subject to local land use regulations and permits. In cities and counties without cultivation regulations of their own, the state shall be the sole licensing authority as of March 1, 2016 (AB 243, 11362.777 (c)4).
TRACK & TRACE PROGAM The DFA shall implement a unique identification program for all marijuana plants at a cultivation site, to be attached at the base of each plant. The information shall be incorporated into a “track and trace” program for each product and transaction. (SB 643, 19335 and AB 243, 11362.777 (e)). Cultivation in violation of these provisions subject to civil penalties up to twice the amount of the license fee, plus applicable criminal penalties. Fines enacted daily for each violation (SB 243, 19360).
PATIENT EXEMPTION Qualified patients are exempt from the state permit program if cultivating less than 100 square feet for personal medical use. Primary caregivers with five or fewer patients are allowed up to 500 square feet (AB 243, 11362.777(g) and SB 643, 19319). Exemption under this section does not prevent a local government from further restricting or banning the cultivation, provision, etc. of medical cannabis by individual patients or caregivers in its jurisdiction (AB 243).
DELIVERIES Cannabis may be delivered to qualified patients only by dispensaries and only in cities or counties where not prohibited by local ordinance. All deliveries to be documented. No locality can bar transport of delivered products through its territory. Deliveries may be taxed by local county. (AB 266, 19340). [In a separate section (19334 (a) 4) it is confusingly stated that dispensers who have no more than three dispensaries (Type 10A) shall be allowed to deliver “where expressly authorized by local ordinance.” It’s unclear what conditions if any apply to other, Type 10 licensed dispensers.]
MANUFACTURERS are to be licensed by DPH. The DPH shall limit the number of Type 7 licenses that produce products using volatile solvents.
TESTING (AB 266, 19341-6) The DPH shall ensure that all cannabis is tested prior to delivery to dispensaries or other businesses, and specify how often such testing shall be conducted. *** Confusingly, 19346(c) says the costs of testing are to be paid by cultivators, whereas 19326(c) (3) states that distributors shall charge for the costs of testing; since distributors serve manufacturers as well as cultivators, it doesn’t make sense that testing costs for the former should be charged to the latter. *** Licensees shall use standard methods established by International Organization for Standardization approved by an accrediting body that is signatory to the International Laboratory Accreditation Cooperation Mutual Recognition Arrangement (AB 266, 19342). Licensees shall test for cannabinoids, contaminants, microbiological impurities, and other compounds spelled out in Section 19344. Licensees may conduct tests for individual qualified patients, but not certify them for resale or transfer to other licensees.
SCHOOL ZONES Cultivation and dispensary facilities must be at least 600 ft from schools (with grandfathered exceptions specified in HSC 11362.768). (SB 643, 19322 (a) 4).
TRANSPORTATION Only licensed transporters can transport cannabis or cannabis products between licensees (AB 266, 19326(a)). The bill doesn’t specify whether cultivators, manufacturers, or retailers can also have transport licenses, but 19328 (a) states they can generally have at most two separate kinds of licenses. Licensed transporters shall transmit an electronic shipping manifest to the state and carry a physical copy with each shipment (SB643, 19337).
LABOR PEACE AGREEMENTS Required of all applicants with 20 employees or more (SB 643, 19322 a (6))
PACKAGING Products shall be labeled in tamper-evident packages with warning statements & information specified in Section 19347.
PRIVACY Identifying names of patients, caregivers, and medical conditions shall be kept confidential. (AB 266, 19355)
SB 420 COLLECTIVE DEFENSE SUNSET The provision in SB 420 affording legal protection to patient collectives and cooperatives, HSC 11362.775, shall sunset one year after the Bureau posts a notice on its website that licenses have commenced being issued. After that date, all cannabis collectives will have to be licensed, except for individual patient and caregiver gardens serving no more than five patients.
PHYSICIAN RECOMMENDATIONS (SB 643): There are several new provisions clarifying the duties of medical cannabis physicians; however, they don’t substantially affect or impair patients’ current access to medical recommendations.
• The Med Board’s enforcement priorities are amended to include “Repeated acts of clearly excessive recommending of cannabis for medical purposes,or repeated acts of recommending without a good faith prior exam.” (SB 643, 2220.05). This is identical to existing language regarding controlled substances, which has generally been assumed to apply to MMJ heretofore.
• It is unlawful for physicians who recommend to accept, solicit, or offer remuneration to or from a licensed facility in which they or a family member have a financial interest.
• The Med Board shall consult with the California Center for Medicinal Cannabis Research in developing medical guidelines for MJ recs.
• The recommending person shall be the patient’s “attending physician” as defined in HSC 11362.7(a). Contrary to popular misconception, this in nothing new and in no way limits patients to their primary care physician. It merely restates current language in SB 420.
• Physician ads must include a warning notice that MMJ is still a federal Schedule One substance.
PESTICIDE STANDARDS shall be promulgated by DFA and Dept of Pesticide Regulation (SB643, 19332).
ORGANIC CERTIFICATION will be made available by DFA by Jan 1, 2020, federal law permitting. (SB643, 19332.5(a))
APPELLATIONS OF ORIGIN T.he bureau MAY establish appellations of origin for cannabis grown in California. No product may be marketed as coming from a county where it was not grown. (SB643, 19332.5(b-d)).
FEES and FUNDING Each licensing authority shall establish a scale of application, licensing and renewal fees, based upon the cost of enforcement. Fees shall be scaled dependent on the size of the business. (AB 243, 19350 (c)). A Medical Marijuana Regulation and Safety Act Fund is established in the state treasury to receive fees and penalties assessed under the act. $10 million is allocated to DCA to begin operations, with the possibility of an additional operating loan of $10 million from the General Fund (AB 243, 19352). The Bureau shall use the fund for a grant program to assist in state and local agencies in enforcement and remediation of environmental impacts from cultivation. (AB 243, 19351)
COUNTY TAXATION Counties may levy a tax on the cultivating, dispensing, producing, processing, distributing, etc, of medical cannabis subject to standard voter approval requirements. (Many cities already exercise this authority, but the authority of counties to do so has been unclear heretofore). (SB 643, 19348)
Text of Medical Marijuana Regulation Safety Act (three parts):
AB 266 (Bonta/Cooley/Jones-Sawyer/Lackey)
AB 243 (Wood)
SB 643 (McGuire)
Sarah Armstrong, Directory of Industry Affairs for Americans for Safe Access, compiled this list of the deadlines in the bills:
July 1, 2015 – Date by which those claiming vertical integration had to be operating a vertically integrated business. (AB 266 Section 19328 (c1))
January 1, 2016 – date on which AB 266, AB 243 and SB 643 will take effect. (See: the end of the legislative summaries in all three bills)
January 1, 2016 – Beginning business operating date for cannabis businesses who are eligible for priority licensing. “In issuing licenses, the licensing authority shall prioritize any facility or entity that can demonstrate to the authority’s satisfaction that it was in operation and in good standing with the local jurisdiction by January 1, 2016.” (AB 266 Section 19321 (c))
March 1, 2016 – Date by which cultivation must be regulated by a locality: “If a city, county, or city and county does not have land use regulations or ordinances regulating or prohibiting the cultivation of marijuana, either expressly or otherwise under principles of permissive zoning, or chooses not to administer a conditional permit program pursuant to this section, then commencing March 1, 2016, the division shall be the sole licensing authority for medical marijuana cultivation applicants in that city, county, or city and county.” (AB 243 Section 19362.777 (c)(4))
January 1, 2017 – By January 1, 2017, the Division of Occupational Safety and Health shall convene an advisory committee to evaluate whether there is a need to develop industry-specific regulations related to the activities of facilities issued a licensee. (AB 266 Labor Code Amendment Sec. 7 147.5)
July 1, 2017 – By July 1, 2017, the advisory committee shall present to the board its findings and recommendations for consideration by the board. (AB 266 Labor Code Amendment Sec. 7 147.5)
July 1, 2017 – By July 1, 2017, the board shall render a decision regarding the adoption of industry-specific regulations pursuant to this section. (AB 266 Labor Code Amendment Sec. 7 147.5)
January 1, 2018 – “a facility or entity that is operating in compliance with local zoning ordinances and other state and local requirements on or before January 1, 2018, may continue its operations until its application for licensure is approved or denied pursuant to this chapter.” (AB 266 Section 19321 (c))
January 1, 2020 – Not later than January 1, 2020, the Department of Food and Agriculture in conjunction with the Bureau, shall make available a certified organic designation and organic certification program for medical marijuana, if permitted under federal law and the National Organic Program. (SB 643 Section 19332.5(a))
January 1, 2022 – Date by which the loan of up to $10,000,000. 00 from the general fund to establish the Medical Marijuana Regulation and Safety Act has to be repaid. If the fees collected by that time don’t repay the loan, they will begin using funds that come from imposing penalties to repay the loan. (AB 243 Section 19351 (b) (1))
March 1, 2023 – Beginning on March 1, 2023, and on or before March 1 of each following year, each licensing authority shall prepare and submit to the Legislature an annual report on the authority’s activities and post the report on the authority’s Internet Web Site. (AB 266 Section 19353)
January 1, 2026 – The date Type 10A Paragraph on licensing become inoperative “A Type 10A licensee may apply for a Type 6 or 7 state license and hold a 1, 1A, 1B, 2, 2A, 2B, 3, 3A, 3B, 4 or combination thereof if, under the 1, 1A, 1B, 2, 2A, 2B, 3, 3A, 3B, 4 or combination of licenses thereof, no more than four acres of total canopy size of cultivation by the licensee is occurring throughout the state during the period that the respective licenses are valid… This paragraph shall become inoperative on January 1, 2026.” ((AB 266 Section 19328 (a) (9))
January 1, 2026 – Date vertical integration section of AB 266 is repealed. (AB 266 Section 19328 (d))